This is what Ernest Rutherford thought when his team discovered that atoms have tiny, positively charged nuclei. Their first thought was that the movement of the electrons prevents them from contacting the nucleus. They had imagined that electrons revolve around the core like small planets revolve around a star, but soon, they realized that everything is a little more complicated.
Read also:
- Why Shouldn’t Marie Curie’s Things Be Touched For At Least Another 1,500 Years?
- Can A Photon Travel Between Two Mirrors Indefinitely?
- Why Is The ISS Located At A Distance Of About 400 km From The Earth? Why Not 600 km, or 1000, or 300?
- Can Asteroids In The Asteroid Belt Gather Together And Become A Planet?
The problem is as follows: according to electrodynamics, when the direction of motion of a charged particle changes, an electromagnetic wave appears, which takes energy from the same particle.
An electron moving in a circle must constantly emit electromagnetic waves, which means that it will lose speed and approach the nucleus until, in the end, it collides with it. Calculations have shown that in this case, the atoms should be destroyed in a split second.
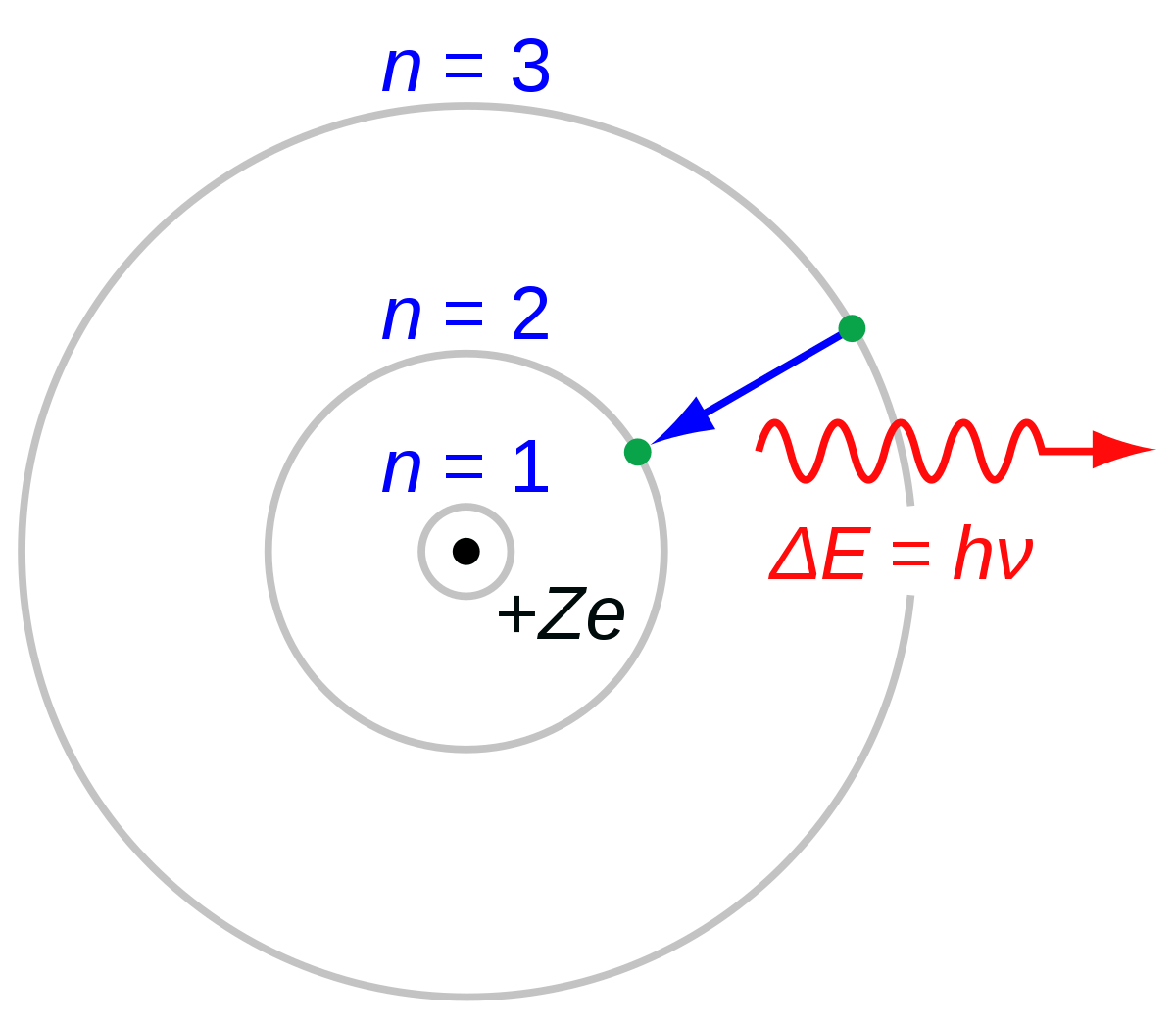
So what’s the deal? Niels Bohr proposed an alternative planetary model of the atom, in which electrons remain in fixed orbits around the nucleus, while in one of these orbits, they do not emit electromagnetic waves.
They can jump from a lower orbit to a higher one if they absorb enough energy: the energy difference between the two orbits. They can also move from a higher orbit to a lower one, emitting an exact amount of electromagnetic energy, again the difference between the two orbits.
It is worth noting that Bohr was not a convincing explanation of why electrons behave in a similar way, but it was easy to demonstrate that it is an adequate approximation to what is happening in reality.
Physicists have long known, that the atoms absorb and emit electromagnetic radiation at a fixed wavelength. Each element has its own spectrum: the color of a light wave, characteristic of this type of atom, but again, no one knew why this was happening (light is a form of electromagnetic radiation).
It took many years to get a more complete theory of electrons in atoms. This theory is called quantum mechanics, and
it is both the most successful and most confusing physics ever invented.
In fact, electrons do not move in neat circular orbits, but in more complex three-dimensional shapes called orbitals.
The Heisenberg Uncertainty Principle is one of the principles of quantum mechanics, which states that the product of the standard spread of position and the standard spread of momentum is greater than or equal to the reduced Planck constant divided by two.
Suppose an electron collides with a nucleus. This means that the position of the electron is estimated with very little deviation. Therefore, as follows from the inequality, there is a large deviation for the electron momentum.
A large spread for momentum means that the momentum is either extremely large in one direction or extremely large in the other. A large impulse is a high speed of rotation and therefore high kinetic energy.
That is, the electron has enough kinetic energy to orbit around the nucleus, thanks to the electromagnetic interactions between them. Consequently, the electron under normal conditions does not collide with the nucleus but is kept at a distance.








